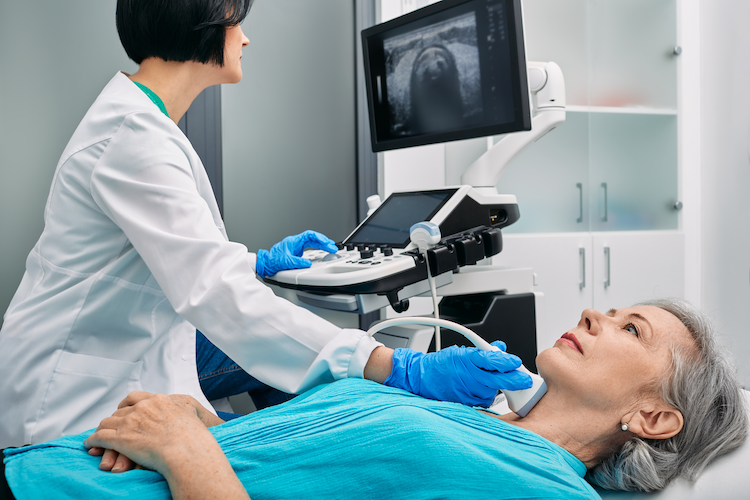
Diagnostics: TI-Rads system for assessing risk of a thyroid nodule by ultrasound
What is The TI-RADS system?
The TI-RADS system, or Thyroid Imaging Reporting and Data System, is a standardized classification system used by radiologists to evaluate the risk of malignancy in thyroid nodules based on ultrasound characteristics. It assigns a score to nodules based on factors such as composition, echogenicity, shape, margin, and the presence of calcifications. Each nodule is categorized into different TI-RADS levels, which range from TR1 (benign) to TR5 (high suspicion of malignancy). This system helps clinicians in making informed decisions about the need for further diagnostic procedures, such as fine-needle aspiration biopsies (FNA)s, by providing a uniform approach to the assessment of thyroid nodules.
Here are the current TI-RADS Recommendations from the American College of Radiology:
TR Level 1 Benign, No FNA.
TR Level 2 Not suspicious. No FNA.
TR Level 3 Mildly suspicious. FNA if >= 2.5 cm. Follow up if >=1.5 cm.
TR Level 4 Moderately suspicious. FNA if > 1.5 cm. Follow up if > 1 cm.
TR Level 5 Highly suspicious. FNA if > 1 cm. Follow up if > 0.5 cm.
Reference: Journal of the American College of Radiology. 2017; Vol. 14; Issue 5: 587-595.
This page